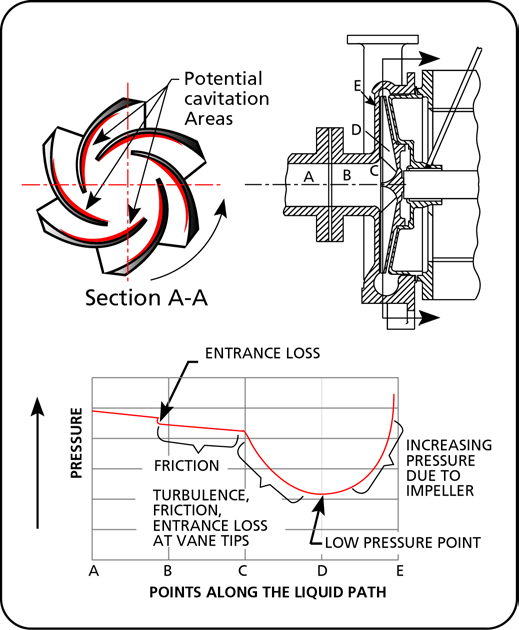
Cavitation begins as the formation of vapor bubbles at the impeller eye due to low pressure. The bubbles form at the position of lowest pressure at the pump inlet (see Figure 1) which is just prior to the fluid being acted upon by the impeller vanes, they are then rapidly compressed. The compression of the vapor bubbles produces a small shock wave that impacts the impeller surface and pits away at the metal creating over time large eroded areas and subsequent failure. The sound of cavitation is very characteristic and resembles the sound of gravel in a concrete mixer.
Figure 1 Pressure profile in a centrifugal pump
As you can see from Figure 1 the pressure available at the pump inlet, which is the pressure that we would measure if we put a gauge at that point, can be reasonably high but still drop considerably as it makes it way into the pump. The pressure may be lowered enough that the fluid will vaporize and will then produce cavitation.
The same effect can sometimes be seen in control valves because they have a similar pressure drop profile, if the pressure is insufficient at the control valve inlet, cavitation will also occur.
Vapor pressure and cavitation
There are two ways to boil a liquid. One way is to increase the temperature while keeping the pressure constant until the temperature is high enough to produce vapor bubbles. In Figure 2 this is what happens if you take one point in the liquid phase and you move horizontally (that is at constant pressure) by increasing the temperature. Eventually you hit the vaporization line of the particular fluid and the fluid starts to boil or produce vapor bubbles.
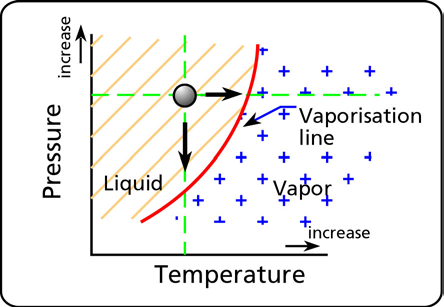
Figure 2 Vapor pressure vs. temperature.
We do he same thing everyday when we boil water in an open pot.
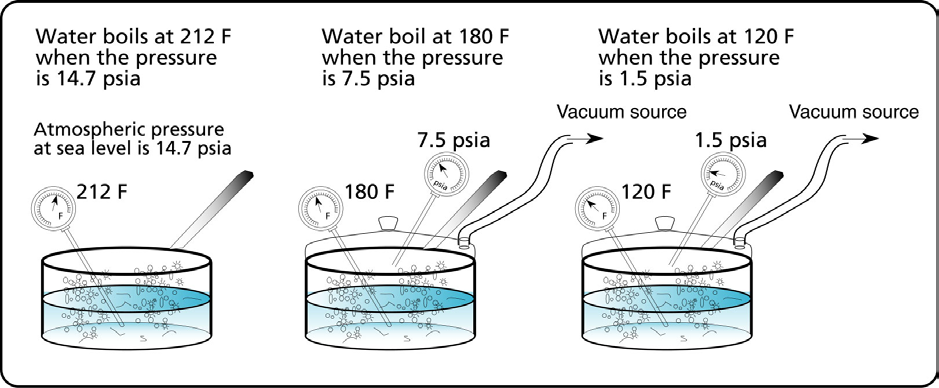
Figure 3 Boiling water with low pressure.
The other way to boil a liquid is to lower the pressure. If you keep the temperature constant and lower the pressure the liquid will also boil. In Figure 2 this is what happens if you take one point in the liquid phase and you move vertically (that is at constant temperature) by decreasing the pressure. Again you hit the vaporization line of the fluid and the fluid starts to boil or produce vapor bubbles.
If the pot is covered and you provide a source of vacuum (see Figure 3), by lowering the pressure in the pot you would be able to make the water boil at a lower temperature.
When the pressure is 14.7 psia or atmospheric pressure at sea level, the temperature required to make water boil is 212 °F. This means that the vapor pressure of water at 212 °F is 14.7 psia. Similarly the vapor pressure of water at 120 °F is 7.5 psia. Vapor pressure is the pressure at which a liquid boils for a given temperature.
It is not unusual for industrial processes to operate at temperatures that are close or higher than 120 F. Therefore if the temperature is high and the pressure drops as the fluid enters the pump, it will be easier to produce cavitation because the pressure drop produced by the pump will have to be smaller to match a higher vapor pressure. If cavitation is occurring or suspected, two possible solutions are: to increase the pressure at the pump inlet or decrease the fluid temperature.

Figure 4 Vapor pressure of various liquids